ENCAB000AOC
Alternate accession: ENCAB000BSG
Antibody against Homo sapiens POLR2A, Mus musculus POLR2A
Homo sapiens
GM12878, K562, HeLa-S3, GM12891, GM12892, body of pancreas, lower leg skin
characterized to standards with exemption
Homo sapiens
any cell type or tissue
partially characterized
Mus musculus
any cell type or tissue
partially characterized
- Status
- released
- Source (vendor)
- Covance
- Product ID
- MMS-126R
- Lot ID
- 14861301
- Characterized targets
- POLR2A (Homo sapiens), POLR2A (Mus musculus)
- Lot ID aliases
- 14922701, E11BF00348
- Host
- mouse
- Clonality
- monoclonal
- Purification
- crude
- Antigen description
- clone 8WG16
- External resources
Characterizations
POLR2A (Homo sapiens)
GM12891GM12892body of pancreaslower leg skin
exempt from standards
- Caption
- Primary characterization (e.g. immunoblot, immunoprecipitation) for the specified cell types and tissues are exempted for this antibody by the ENCODE antibody review panel because it is a well-characterized monoclonal antibody that has been in use without issue by the community for many years.
- Submitter comment
- This is a well characterized antibody already used in many ENCODE experiments.
- Reviewer comment
- Primary characterizations for this antibody were exempted for all cell types and tissues by the Antibody Review Panel on March 30, 2016.
- Submitted by
- Esther Chan
- Lab
- J. Michael Cherry, Stanford
- Grant
- U41HG006992
- Download
- exempted.png
POLR2A (Homo sapiens)
Method: immunoprecipitation followed by mass spectrometry
not reviewed
- Submitted by
- Michael Snyder
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG004558
- Download
- human_Pol2_validation_Snyder.pdf
POLR2A (Homo sapiens)
Method: immunoprecipitation
not reviewed
- Caption
- A single band consistent with the mobility expected for POLR2A/RPB1 (~217kD) is observed in nuclear extracts from cell lines K562 and HeLa S3 (Panel A) and cell line GM12878 (Panel B). This band is efficiently and specifically immunoprecipitated from GM12878 nuclear extract by antibody MMS-126R (Panel B). Analysis by mass spectrometry of material immunoprecipitated by MMS-126R from HeLaS3 cells shows very strong enrichment for POLR2A and associated components of RNA polymerase II (see validation assay #2). Therefore, MMS-126R meets this criterion for validation.
- Submitted by
- Michael Snyder
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG004558
- Download
- human_Pol2_validation_Snyder.pdf
POLR2A (Homo sapiens)
GM12878
compliant
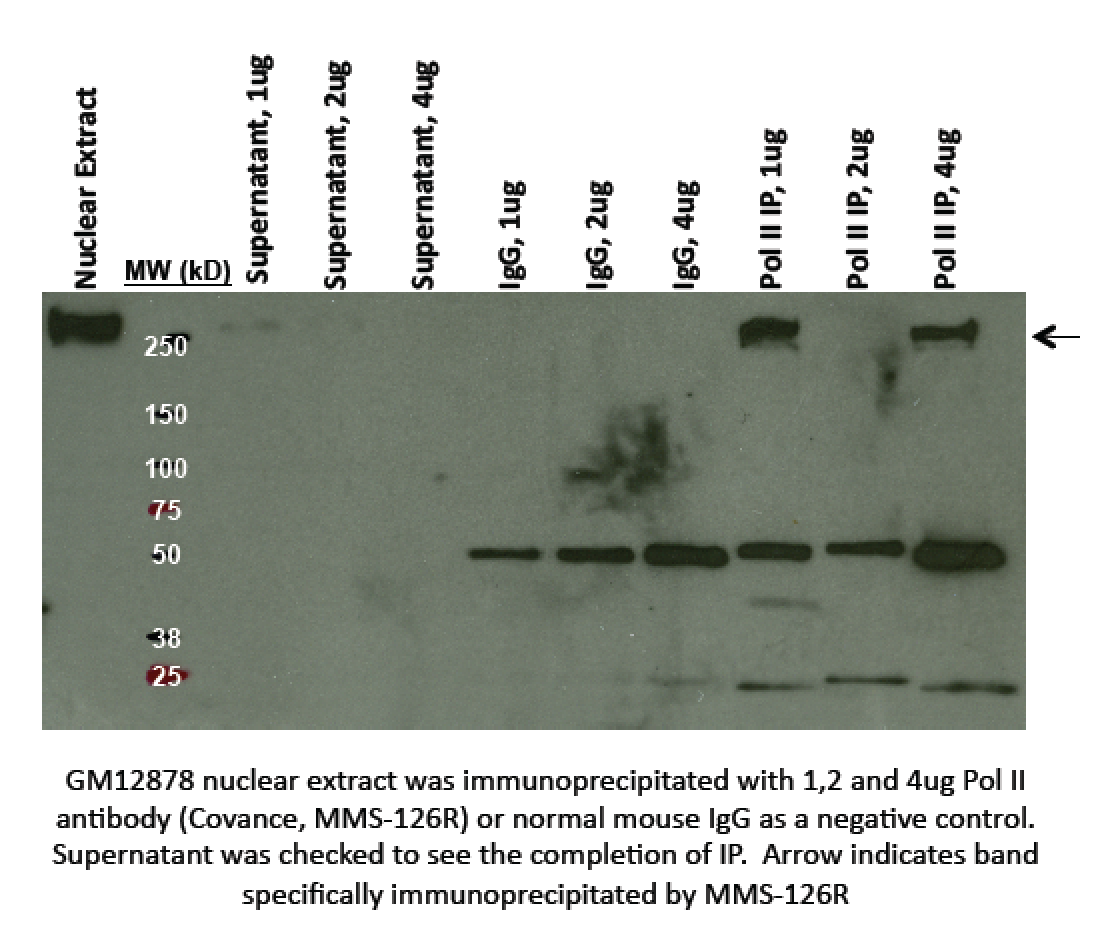
- Caption
- Immunoprecipitation: GM12878 nuclear extract was immunoprecipitated with 1,2 and 4ug Pol II antibody (Covance, MMS‐126R) or normal mouse IgG as a negative control. Supernatant was checked to see the completion of IP. Arrow indicates band specifically immunoprecipitated by MMS‐126R (expected band size ~217kD). The lane found compliant is lane 9, labelled "Pol II IP, 4ug".
- Submitted by
- Kathrina Onate
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG004558
- Download
- IP POLR2A AOC Snyder.png
POLR2A (Mus musculus)
Method: immunoprecipitation followed by mass spectrometry
not reviewed
- Submitted by
- Michael Snyder
- Lab
- Michael Snyder, Stanford
- Grant
- RC2HG005602
- Download
- mouse_Pol2_validation_Snyder.pdf
POLR2A (Mus musculus)
Method: immunoprecipitation
not reviewed
- Caption
- Immunoprecipitation of POLR2A from HeLa S3 cells using MMS-126R followed by mass spectrometry: Briefly, HeLaS3 whole cell lysates were immunoprecipitated using MMS-126R ,and the IP fraction was loaded on a 10% polyacrylamide gel (NuPAGE Bis-Tris Gel) and separated with an invitrogen NuPAGE electrophoresis system. The gel was silver-stained, gel fragments corresponding the full length of the gel were excised and destained using the SilverSNAP Stain for Mass Spectrometry (Pierce). Then proteins were trysinized using the in-gel digestion method. Digested proteins were analyzed on an LTQ-Orbitrap (Thermo Scientific) by the nanoLC-ESI-MS/MS technique. Peptides were identified by the SEQUEST algorithm and filtered with a high confidence threshold (Protein false discovery rate < 1%, 2 peptides per protein minimum). Samples were run twice and results from both runs are presented We report 158 proteins identified in this immunoprecipitation. However, only 21 of these were identified in the immunoprecipitation with MMS-126R, but not the control immunoprecipitation. Of these 21, POLR2A is the most abundant (highlighted in yellow) and all other abundantly identifed proteins (highlighted in blue) are known to be part of the RNA polymerase II complex . Based on these observations, MMS-126R meets the ENCODE standard for validation by this criterion.
- Submitted by
- Michael Snyder
- Lab
- Michael Snyder, Stanford
- Grant
- RC2HG005602
- Download
- mouse_Pol2_validation_Snyder.pdf
POLR2A (Homo sapiens)
Method: immunoprecipitation followed by mass spectrometry
exempt from standards
- Caption
- Immunoprecipitation of POLR2A from HeLa-S3 cells using MMS-126R followed by mass spectrometry. Briefly, HeLa-S3 whole cell lysates were immunoprecipitated using MMS-126R, and the IP fraction was loaded on a 10% polyacrylamide gel (NuPAGE Bis-Tris Gel) and separated with an Invitrogen NuPAGE electrophoresis system. The gel was silver-stained, gel fragments corresponding to the full length of the gel were excised and destained using the SilverSNAP Stain for Mass Spectrometry (Pierce). Then, proteins were trypsinized using the in-gel digestion method. Digested proteins were analyzed on an LTQ-Orbitrap (Thermo Scientific) by the nanoLC-ESI-MS/MS technique. Peptides were identified by the SEQUEST algorithm and filtered with a high confidence threshold (Protein false discovery rate < 1%, 2 peptides per protein minimum). Samples were run twice and results from both runs are presented.
- Submitter comment
- This characterization was done a long time ago and an image of the IP before mass-spec and the full mass-spec peptide table are no longer available to submit.
- Reviewer comment
- The lack of an image of the IP with the excised band(s) marked was OK'd by the ENCODE antibody review panel (03/28/16).
- Submitted by
- Minyi Shi
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG006996
- Download
- Poll2_mass_spec.pdf
POLR2A (Homo sapiens)
K562HeLa-S3
compliant
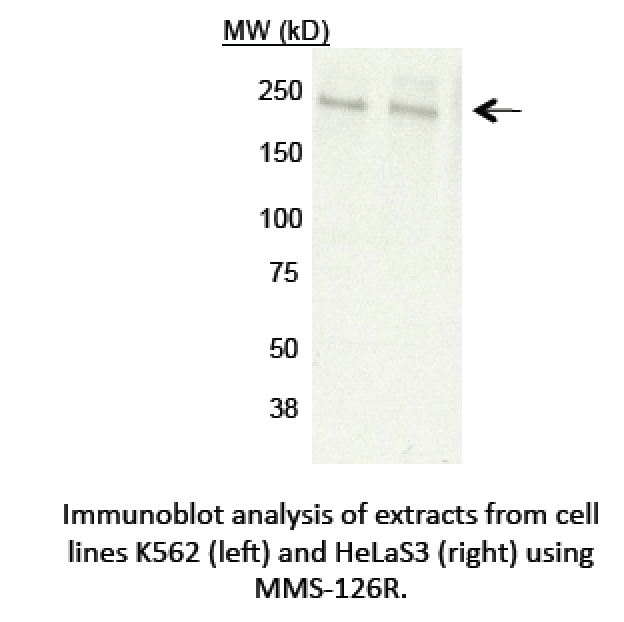
- Caption
- Immunoblotting: A single band consistent with the mobility expected for POLR2A/RPB1 (~217kD) is observed in nuclear extracts from cell lines K562 and HeLa S3 and cell line GM12878. This band, labeled by the arrow, is efficiently and specifically immunoprecipitated from GM12878 nuclear extract by antibody MMS-126R.
- Submitted by
- Kathrina Onate
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG004558
- Download
- WB POLR2A AOC Snyder.png