ENCAB691BTB
Antibody against Homo sapiens HDGF
Homo sapiens
K562
characterized to standards
- Status
- released
- Source (vendor)
- Bethyl Labs
- Product ID
- A303-169A
- Lot ID
- 1
- Characterized targets
- HDGF (Homo sapiens)
- Host
- rabbit
- Clonality
- polyclonal
- Purification
- affinity
- Aliases
- michael-snyder:AS-1026
- External resources
Characterizations
HDGF (Homo sapiens)
K562
exempt from standards
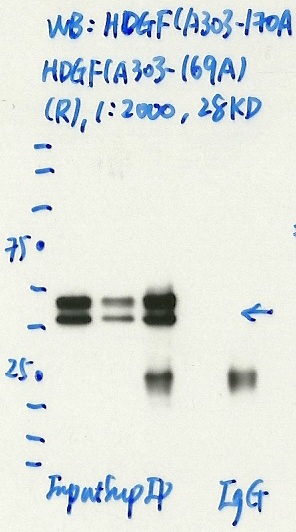
- Caption
- Immunoprecipitation was performed on nuclear extracts from the cell line: K562, using the antibody A303-169A. The blot shows western blot analysis of input, flowthrough, immunoprecipitate and mock immunoprecipitate using IgG.
- Submitter comment
- HDGF is known to form a domain-swapped homodimer that can be stable even in reducing gels (PMID: 17270212)
- Reviewer comment
- Higher than expected size and multiple bands were noted. In response to the submitter's comment, the stable domain-swapped homodimer would explain the higher (double-sized) band and also explain why the ratio of the two bands can be different on different westerns.
- Submitted by
- Denis Salins
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG006996
HDGF (Homo sapiens)
K562
compliant
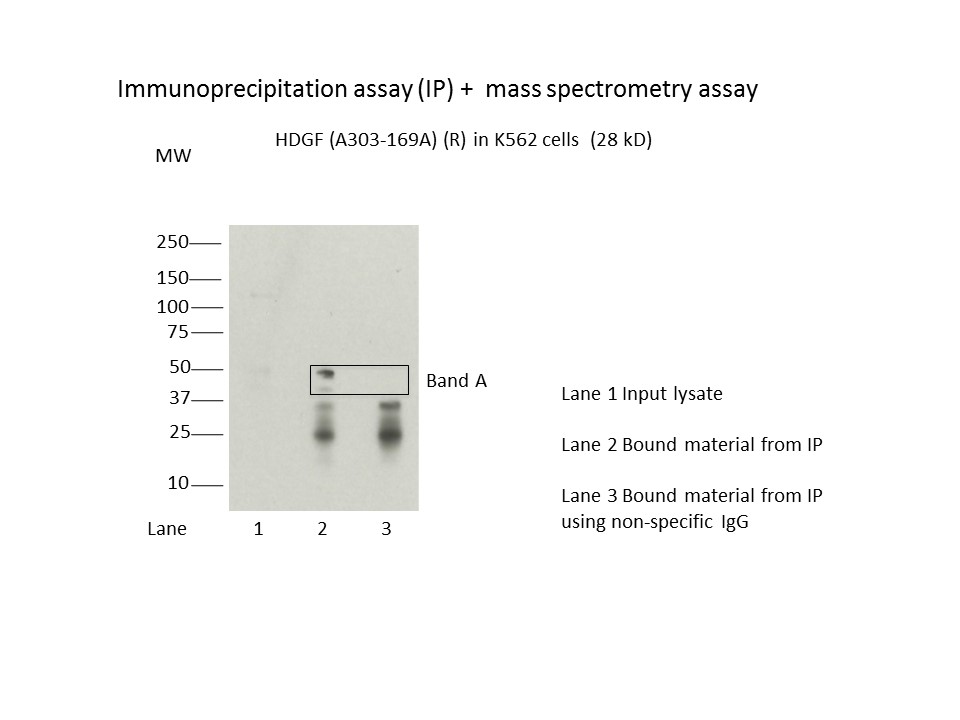
- Caption
- Immunoprecipitation of HDGF from K562 cells using A303-169A. Lane 1: input nuclear lysate. Lane 2: material immunoprecipitated with A303-169A. Lane 3: material immunoprecipitated using control IgG. Band A was excised from gel and subject to analysis by mass spectrometry. The expected band size is 28 kDa.
- Submitted by
- Kathrina Onate
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG006996
- Download
- HDGF.jpeg
HDGF (Homo sapiens)
Method: immunoprecipitation followed by mass spectrometry
compliant
- Caption
- IP followed by mass spectrometry: Briefly, protein was immunoprecipitated from K562 nuclear cell lysates using A303-169A, and the IP fraction was loaded on a 10% polyacrylamide gel (NuPAGEBis-Tris Gel) and separated with an Invitrogen NuPAGE electrophoresis system. The gel was stained by ColloidialCoomassie G-250 stain, gel fragments corresponding to the bands indicated were excised. Then proteins were trypsinized using the in-gel digestion method. Digested proteins were analyzed on an Orbitrap Elite mass spectrometer (Thermo Scientific) by the nanoLC-ESI-MS/MS technique. Peptides were identified by the SEQUEST algorithm and filtered with a high confidence threshold (Peptide false discovery rate < 1%, 2 unique peptides per protein minimum, mass error < 10 ppm).
- Submitted by
- Kathrina Onate
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG006996
- Download
- HDGF_A303-169A final.pdf