ENCAB000ASX
Antibody against Homo sapiens EGR1
Homo sapiens
K562, GM12878, MCF-7
characterized to standards
Homo sapiens
liver
characterized to standards with exemption
Homo sapiens
HepG2
not characterized to standards
- Status
- released
- Source (vendor)
- Santa Cruz Biotech
- Product ID
- sc-110
- Lot ID
- unknown
- Characterized targets
- EGR1 (Homo sapiens)
- Host
- rabbit
- Clonality
- polyclonal
- External resources
Characterizations
EGR1 (Homo sapiens)
K562GM12878HepG2MCF-7
compliant
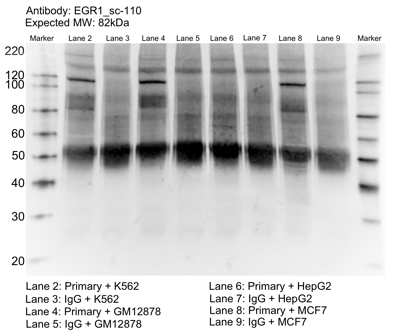
- Caption
- Whole cell lysates of K562, MCF7, GM12878 and HepG2 were immunoprecipitated using the primary antibody (Santa Cruz Biotechnology; sc-110). The IP fraction was separated on a 12% acrylamide gel with the Bio-Rad PROTEAN II xi system. After separation, the samples were transferred to a nitrocellulose membrane with an Invitrogen iBlot system. The membrane was probed with the primary antibody (same as that used for IP) and a secondary HRP-conjugated antibody. The resulting bands were visualized with SuperSignal West Femto Solution (Thermo Scientific). Protein Marker (PM) is labeled in kDa. The approximate size of EGR1 is ~82 kDa.
- Reviewer comment
- Band is missing for HepG2
- Submitted by
- Mark Mackiewicz
- Lab
- Richard Myers, HAIB
- Grant
- U54HG006998
- Download
- EGR1_sc-110_092916_L.png
EGR1 (Homo sapiens)
K562
not compliant
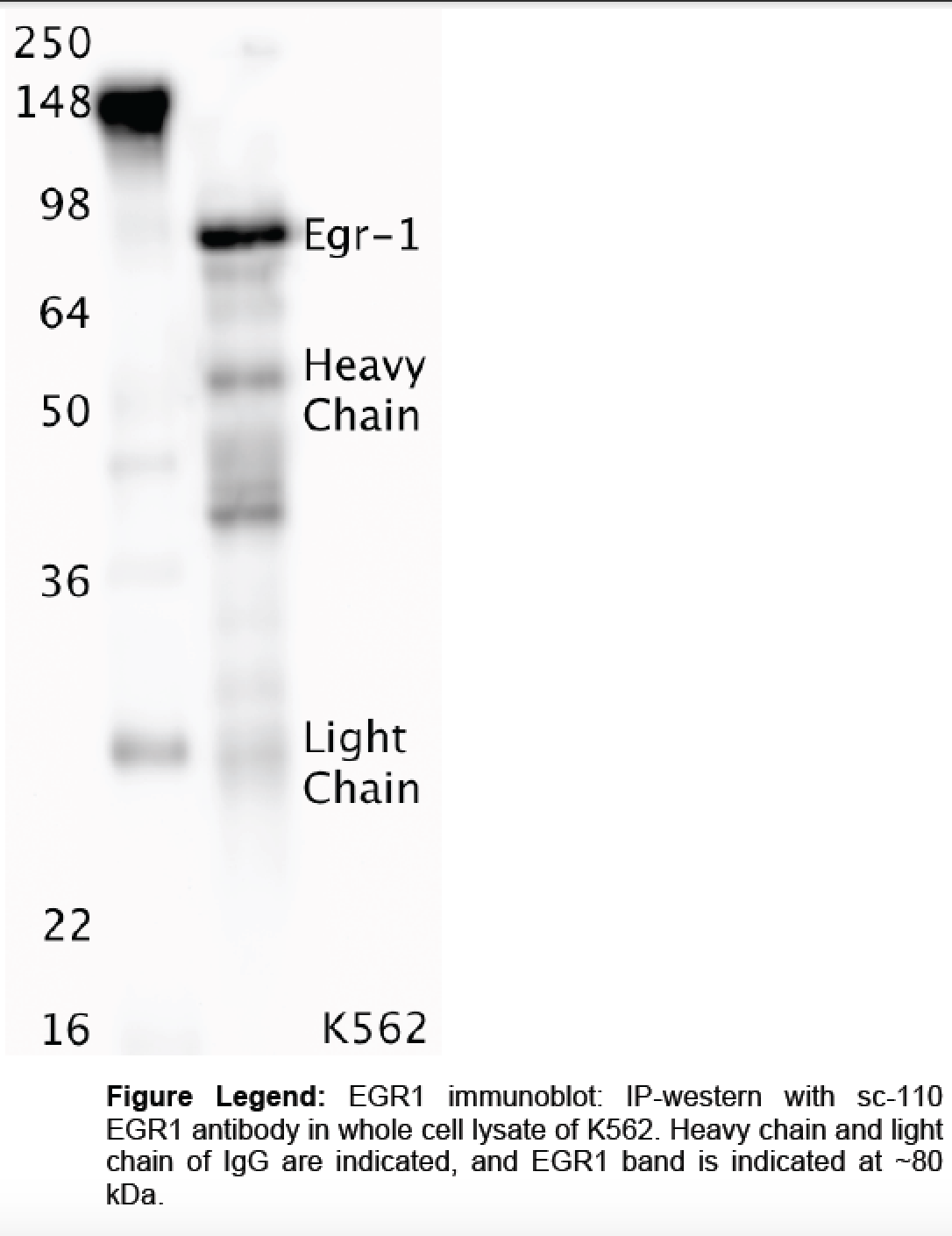
- Caption
- Whole cell lysates were immunoprecipitated using primary antibody, and the IP fraction was loaded on a 12% acrylamide gel and separated with a Bio-Rad PROTEAN II xi system. After separation, the samples were transferred to a nitrocellulose membrane using a Bio-Rad Trans-Blot Electrophoretic Transfer system. Standard western blot protocol was used to probe the membrane with the primary antibody (same antibody as used for IP), and an HRP-conjugated secondary antibody and SuperSignal West Femto solution (Thermo Scientific) were used to detect the immunoprecipitated proteins.
- Reviewer comment
- According to Uniprot, the expected size of the protein is ~57.5 kDa. The indicated band is not in that size range, and the one within that size range (i.e. "heavy chain") is not 50% of the overall lane signal.
- Submitted by
- Richard Myers
- Lab
- Richard Myers, HAIB
- Grant
- U54HG004576
EGR1 (Homo sapiens)
compliant
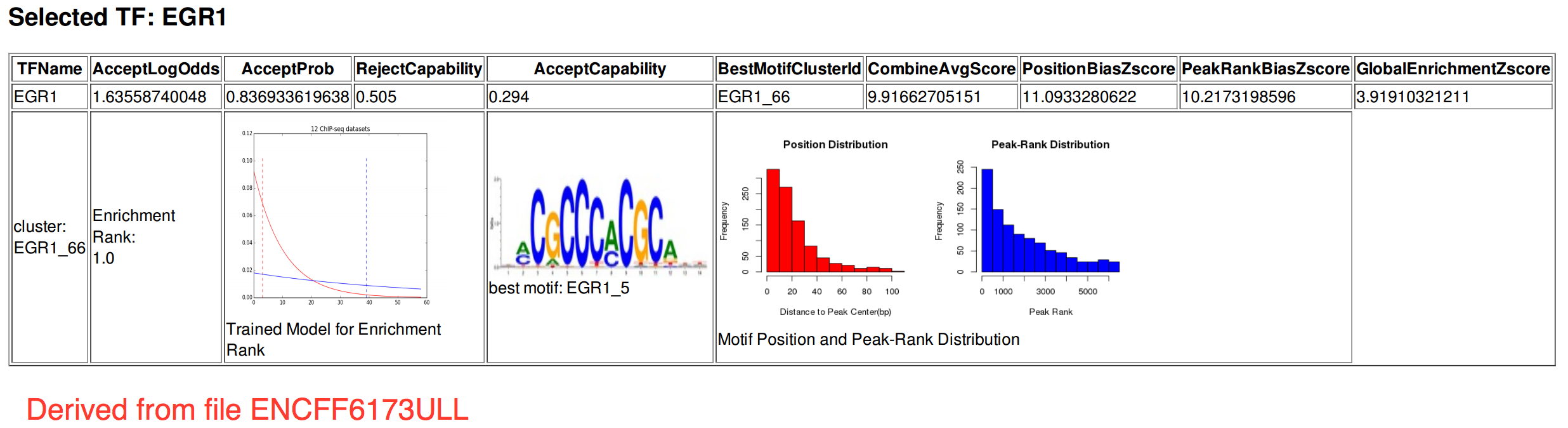
- Caption
- The motif for target EGR-1 is represented by the attached position weight matrix (PWM) derived from files ENCFF686RWF and ENCFF173ULL. Motif enrichment analysis was done by Dr. Zhizhuo Zhang (Broad Institute, Kellis Lab) using known motifs (http://compbio.mit.edu/encode-motifs/) and previously published ChIP-seq data (http://www.broadinstitute.org/~zzhang/motifpipeline/data/TrainSetInfo.txt). The accept probability score of the given transcription factor was calculated using a Bayesian approach. This analysis also includes three motif enrichment scores, computed by overlapping the motif instances with the given ChIP-seq peak locations. For more information on the underlying statistical methods, please see the attached document. From ENCFF617ULL: Accept probability score: 0.836933619638, Global enrichment Z-score: 3.91910321211, Positional bias Z-score: 11.0933280622, Peak rank bias Z-score: 10.2173198596. From ENCFF686RWF: Accept probability score: 0.836933619638, Global enrichment Z-score: 3.49060733105, Positional bias Z-score: 9.74981978772, Peak rank bias Z-score: 10.7393276496, Enrichment rank: 1.0.
- Submitted by
- Aditi Narayanan
- Lab
- Richard Myers, HAIB
- Grant
- U54HG006998
EGR1 (Homo sapiens)
liver
exempt from standards
- Caption
- The ENCODE Binding Working Group finds for some valuable tissues that recreating a primary on well characterized antibodies is not cost effective. Therefore, they allow exemption from standards for these tissues.
- Submitter comment
- The lab is asking for an exemption for liver cells due to the lack of resource to make a primary characterization for them
- Reviewer comment
- Could not be further characterized due to lack of biosample but exempted as two compliant characterizations in other tissue/cell types were provided as per the ENCODE antibody characterization standards.
- Submitted by
- Richard Myers
- Lab
- Richard Myers, HAIB
- Grant
- U54HG006998
- Download
- No_tissue.png