ENCAB000AQM
Antibody against Mus musculus H3K27ac
Homo sapiens
any cell type or tissue
characterized to standards with exemption
Mus musculus
any cell type or tissue
characterized to standards with exemption
- Status
- released
- Source (vendor)
- Active Motif
- Product ID
- 39133
- Lot ID
- 21311004
- Characterized targets
- H3K27ac (Mus musculus)
- Host
- rabbit
- Clonality
- polyclonal
- Purification
- Protein A
- Isotype
- IgG
- Antigen description
- Histone H3 acetyl Lys27 antibody was raised against a peptide including acetyl-lysine 27 of histone H3
- External resources
Characterizations
H3K27ac (Mus musculus)
exempt from standards
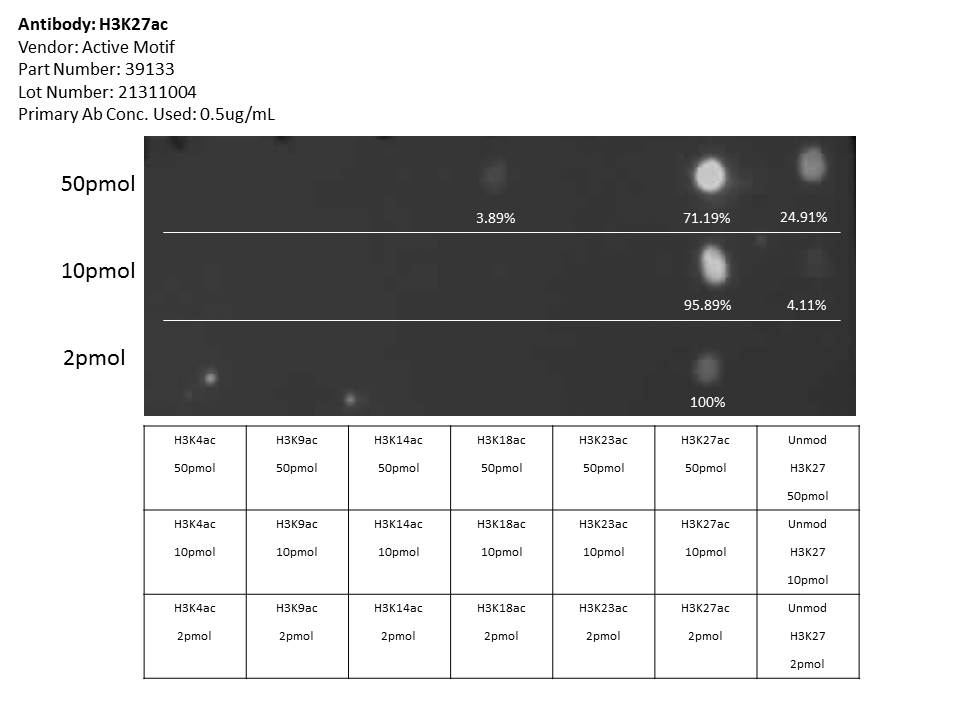
- Caption
- Dot blot analysis performed by Active Motif to confirm the specificity of Histone H3 acetyl Lys27 antibody (cat#39133, lot#21311004) for acetyl Lys27 histone H3. Peptides were spotted onto the membrane in the following concentrations: 50pmol (top row), 10pmol (middle row), 2pmol (bottom row). The blot was then probed with the antibody at 0.5ug/mL. The antibody recognized the H3K27ac peptide with >10-fold enrichment in binding signal relative to other peptides when peptides were used at 10pmol and 2pmol. At 50pmol, the antibody showed a 2.5-fold enrichment of H3K27ac relative to unmodified H3K27 and >10-fold enrichment of H3K27ac relative to H3K18ac.
- Submitter comment
- There is no more of this antibody lot to carry out an additional dot-blot, but there are other H3K27ac antibodies from Active Motif used by this lab which have shown similar ChIP-seq and characterization results.
- Reviewer comment
- We agree that this antibody should be approved via exemption due to lack of additional antibody lot to test and comparable results with other H3K27ac antibodies.
- Submitted by
- Jean Davidson
- Lab
- Bing Ren, UCSD
- Grant
- U54HG006997
- Download
- H3K27ac ENCAB000AQM DotBlot.jpg
H3K27ac (Mus musculus)
A375embryonic fibroblastHEK293ES-Bruce4
compliant
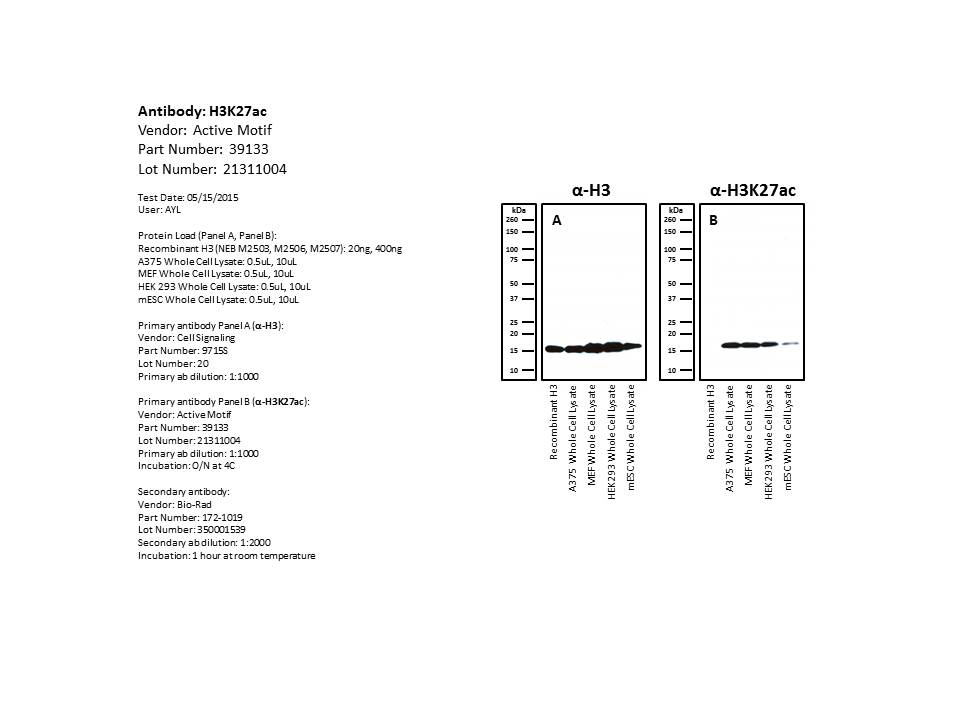
- Caption
- An immunoblot using whole cell extract of two human cell lines and two mouse cell lines shows positive bands at the size of the histone (17kDa), while the recombinant unmodified version of that histone shows a lack of reactivity. The histone bands detected in the extracts constitute 100% of the protein signal and show at least 10-fold enrichment relative to any other band. This signal is also at least 10-fold enriched relative to that detected using the unmodified recombinant histone.
- Submitted by
- David Gorkin
- Lab
- Bing Ren, UCSD
- Grant
- U54HG006997
- Download
- H3K27ac ENCAB000AQM Western.jpg