ENCAB000AMP
Antibody against Homo sapiens ZKSCAN1, Mus musculus ZKSCAN1
Homo sapiens
MCF-7, K562, HepG2, HEK293T
characterized to standards
Homo sapiens
any cell type or tissue
partially characterized
Mus musculus
any cell type or tissue
partially characterized
Homo sapiens
GM12878, HeLa-S3
not characterized to standards
- Status
- released
- Source (vendor)
- Sigma
- Product ID
- HPA006672
- Lot ID
- A44630
- Characterized targets
- ZKSCAN1 (Homo sapiens), ZKSCAN1 (Mus musculus)
- Host
- rabbit
- Clonality
- polyclonal
- Purification
- affinity
- Antigen description
- Immunogen: Zinc finger with KRAB and SCAN domain-containing protein 1 recombinant protein epitope signature tag (PrEST).
- External resources
Characterizations
ZKSCAN1 (Homo sapiens)
not submitted for review by lab
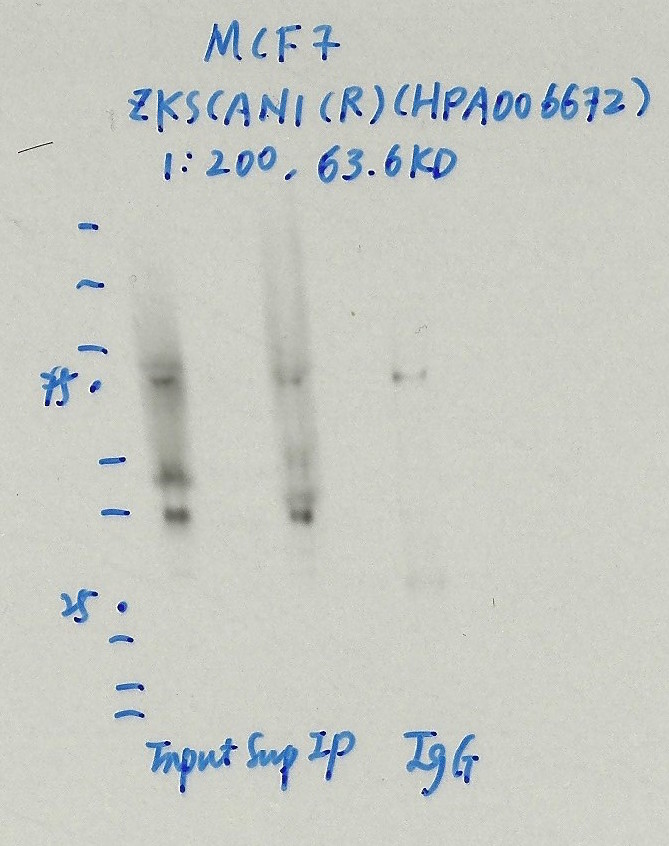
- Caption
- Immunoprecipitation was performed on nuclear extracts from the cell line: MCF-7, using the antibody HPA006672. The blot shows western blot analysis of input, flowthrough, immunoprecipitate and mock immunoprecipitate using IgG.Molecular Weight: 63.6
- Submitted by
- Nathaniel Watson
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG006996
- Download
- 1128_04_ZKSCAN1_HPA006672.jpg
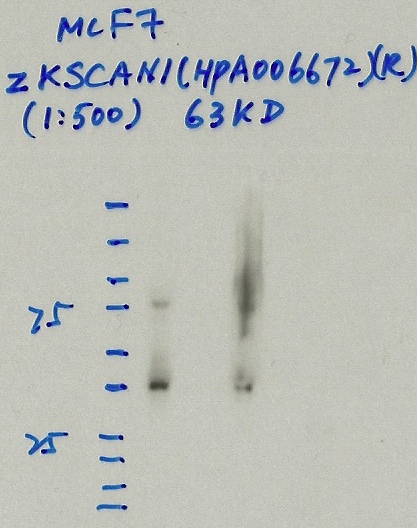
ZKSCAN1 (Homo sapiens)
MCF-7
not compliant
- Caption
- Immunoprecipitation was performed on nuclear extracts from the cell line: MCF-7, using the antibody HPA006672. The blot shows western blot analysis of input, flowthrough, immunoprecipitate and mock immunoprecipitate using IgG.
- Reviewer comment
- No clear band.
- Submitted by
- Denis Salins
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG006996
- Download
- 814IP_5 ZKSCAN1.jpg
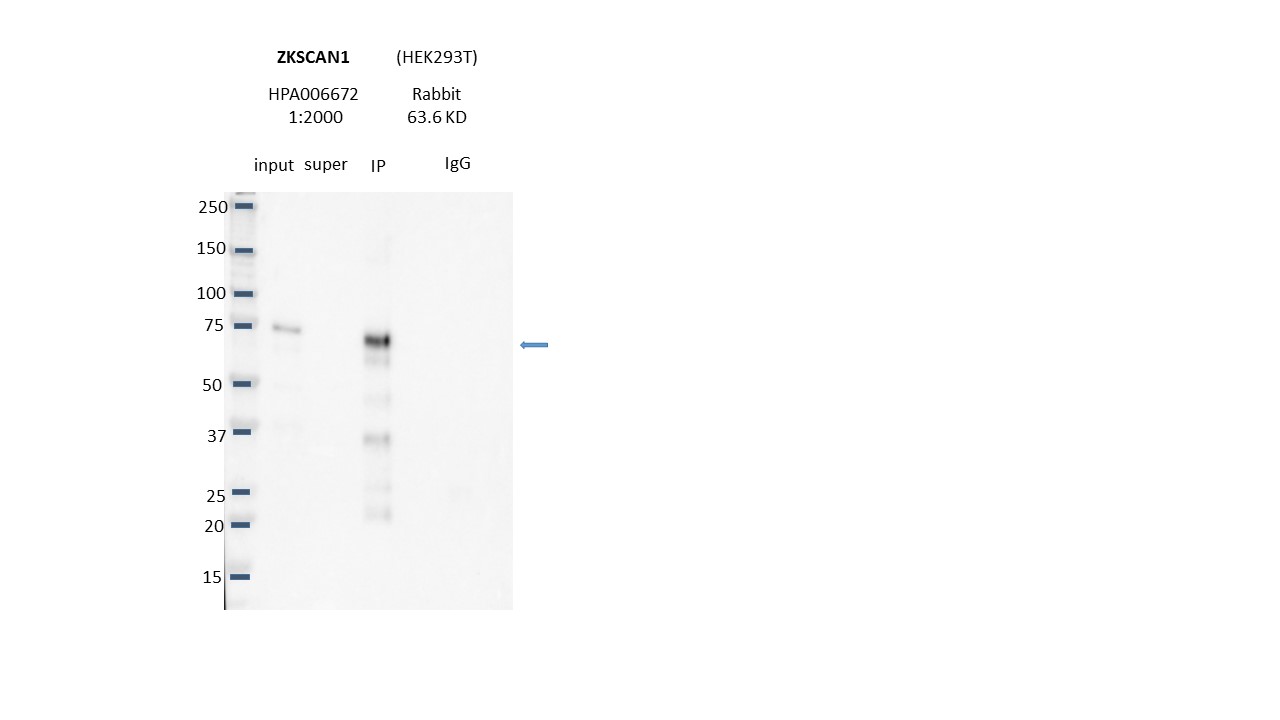
ZKSCAN1 (Homo sapiens)
HEK293T
not compliant
- Caption
- Immunoprecipitation was performed on nuclear extracts from the cell line: HEK293T, using the antibody HPA006672. The blot shows western blot analysis of input, flowthrough, immunoprecipitate and mock immunoprecipitate using IgG.Molecular Weight: 63.6
- Reviewer comment
- KCO: Expected band is not >50%
- Submitted by
- Nathaniel Watson
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG006996
- Download
- Expt1119_2-ZKSCAN1-HPA006672.JPG
ZKSCAN1 (Homo sapiens)
Method: immunoprecipitation followed by mass spectrometry
not reviewed
- Caption
- In an immunoblot probed with HPA006672, we observe a major band consistent with the expected size of ZKSCAN1 (63kD) in cell lines GM12878, K562, HeLaS3, and HepG2 (Panel A). We note an additional significant band of ~60kD in K562, HeLas3, and HepG2 cells. However, while the 63kD band is specifically immunoprecipitated from HepG2 cells, the 60kD band is not (Panel B). Mass spectrometry analysis verified the presence of ZKSCAN1 as the major component of the primary band, as well as of minor low molecular weight bands (see second validation). Therefore, HPA006672 meets this criterion for validation.
- Submitted by
- Michael Snyder
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG004558
ZKSCAN1 (Homo sapiens)
Method: immunoprecipitation
not reviewed
- Caption
- In an immunoblot probed with HPA006672, we observe a major band consistent with the expected size of ZKSCAN1 (63kD) in cell lines GM12878, K562, HeLaS3, and HepG2 (Panel A). We note an additional significant band of ~60kD in K562, HeLas3, and HepG2 cells. However, while the 63kD band is specifically immunoprecipitated from HepG2 cells, the 60kD band is not (Panel B). Mass spectrometry analysis verified the presence of ZKSCAN1 as the major component of the primary band, as well as of minor low molecular weight bands (see second validation). Therefore, HPA006672 meets this criterion for validation.
- Submitted by
- Michael Snyder
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG004558
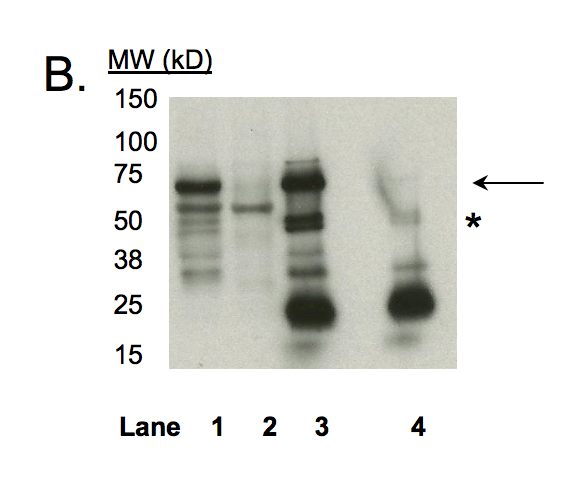
ZKSCAN1 (Homo sapiens)
HepG2
compliant
- Caption
- In an immunoblot probed with HPA006672, we observe a major band consistent with the expected size of ZKSCAN1 (63kD) in cell lines GM12878, K562, HeLaS3, and HepG2 (Panel A). We note an additional significant band of ~60kD in K562, HeLas3, and HepG2 cells. However, while the 63kD band is specifically immunoprecipitated from HepG2 cells, the 60kD band is not (Panel B). Mass spectrometry analysis verified the presence of ZKSCAN1 as the major component of the primary band, as well as of minor low molecular weight bands (see second validation). Therefore, HPA006672 meets this criterion for validation. Immunoprecipitation was performed on nuclear lysates from HepG2 cells using antibody HPA006672. Lane 1: Nuclear lysate. Lane 2: Unbound material from immunoprecipitation with HPA006672. Lane 3: Bound material from immunoprecipitation with HPA006672. Lane 4: Bound material from control immunoprecipitation with rabbit IgG. Arrow indicates band of expected size (63.6 kD) that is highly enriched in the specifically immunoprecipitated fraction. Asterisk indicates signal due to IgG heavy chain from immunoprecipitation.
- Submitted by
- Kathrina Onate
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG004558
- Download
- IP Snyder AMP1.png
ZKSCAN1 (Homo sapiens)
K562
compliant
- Caption
- Immunoprecipitation of ZKSCAN1 from K562 cells using HPA006672. Lane 1: input nuclear lysate. Lane 2: material immunoprecipitated with ab87525. Lane 3: material immunoprecipitated using control IgG. Band A, the most abundant band and consistent with the expected size of ZKSCAN1, along with minor bands B and C were excised from the gel and subject to analysis by mass spectrometry.
- Submitted by
- Kathrina Onate
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG004558
- Download
- IP-MS_ZKSCAN1:K562 Snyder AMP.png
ZKSCAN1 (Mus musculus)
Method: immunoprecipitation
not reviewed
- Caption
- Immunoprecipitation of CH12 and MEL nuclear extracts using anti-ZKSCAN1 antibody (HPA006672) efficiently enriched a single band of the expected molecular weight of ZKSCAN1(~60 kD).
- Submitted by
- Michael Snyder
- Lab
- Michael Snyder, Stanford
- Grant
- RC2HG005602
- Download
- mouse_ZKSCAN1_validation_Snyder.pdf
ZKSCAN1 (Mus musculus)
Method: immunoprecipitation followed by mass spectrometry
not reviewed
- Caption
- Immunoprecipitation of ZKSCAN1 from K562 cells using HPA006672. Lane 1: input nuclear lysate, Lane 2: material immunoprecipitated with ab87525, Lane 3: material immunoprecipitated using control IgG. Band A, the most abundant band and consisent with the expected size of ZKSCAN1, along with minor bands B and C were excised from the gel and subject to analysis by mass spectrometry. IP followed by mass spectrometry: Briefly, K562 whole cell lysates were immunoprecipitated using HPA006672, and the IP fraction was loaded on a 10% polyacrylamide gel (NuPAGE Bis-Tris Gel) and separated with an invitrogen NuPAGE electrophoresis system. The gel was silver-stained, gel fragments corresponding to the bands indicated were excised and destained using the SilverSNAP Stain for Mass Spectrometry (Pierce). Then proteins were trypsinized using the in-gel digestion method. Digested proteins were analyzed on an LTQ-Orbitrap (Thermo Scientific) by the nanoLC-ESI-MS/MS technique. Peptides were identified by the SEQUEST algorithm and filtered with a high confidence threshold (Protein false discovery rate < 1%, 2 peptides per protein minimum). We report 21 proteins identified in band A. ZKSCAN1 is by far the most abundant of these (49 peptides) and is not detected in the IgG specificity control. ZKSCAN1 is also the most abundant protein identifed in minor bands B (7 peptides) and C (10 peptides). Mass spectrometry identifies ZKSCAN1 in levels that are consistent with the relative intensities of the bands in the immunoblot. Based on these observations, the major band is likely due to the presence of immunoprecipated ZKSCAN1 and HPA006672 meets the ENCODE standard for validation by this criterion.
- Submitted by
- Michael Snyder
- Lab
- Michael Snyder, Stanford
- Grant
- RC2HG005602
- Download
- mouse_ZKSCAN1_validation_Snyder.pdf
ZKSCAN1 (Homo sapiens)
GM12878K562HeLa-S3HepG2
compliant
- Caption
- In an immunoblot probed with HPA006672, we observe a major band consistent with the expected size of ZKSCAN1 (63kD) in cell lines GM12878, K562, HeLaS3, and HepG2 (Panel A). We note an additional significant band of ~60kD in K562, HeLas3, and HepG2 cells. However, while the 63kD band is specifically immunoprecipitated from HepG2 cells, the 60kD band is not (Panel B). Mass spectrometry analysis verified the presence of ZKSCAN1 as the major component of the primary band, as well as of minor low molecular weight bands (see second validation). Therefore, HPA006672 meets this criterion for validation. Western blot using antibody HPA0006672 on nuclear lysates from cell lines GM12878 (Lane 1), K562 (Lane 2), HeLa-S3 (Lane 3), and HepG2 (Lane 4).
- Submitted by
- Kathrina Onate
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG004558
- Download
- WB Snyder AMP.png
ZKSCAN1 (Homo sapiens)
HEK293T
compliant
- Caption
- Immunoprecipitation was performed on nuclear extracts from the cell line: HEK293T using the antibody HPA006672. The image shows western blot analysis of input, flowthrough, immunoprecipitate, and mock immunoprecipitate using IgG. Target molecular weight: 63.6.
- Submitted by
- Nathaniel Watson
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG006996
- Download
- ZKSCAN1-HPA006672.jpg
ZKSCAN1 (Homo sapiens)
MCF-7
compliant
- Caption
- Immunoprecipitation was performed on nuclear extracts from the cell line: MCF-7, using the antibody HPA006672. The blot shows western blot analysis of input, flowthrough, immunoprecipitate and mock immunoprecipitate using IgG.Molecular Weight: 63.6
- Submitted by
- Nathaniel Watson
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG006996
- Download
- ZKSCAN1.JPG
ZKSCAN1 (Homo sapiens)
Method: immunoprecipitation followed by mass spectrometry
compliant
- Caption
- IP followed by mass spectrometry: Briefly, K562 whole cell lysates were immunoprecipitated using HPA006672, and the IP fraction was loaded on a 10% polyacrylamide gel (NuPAGEィ Bis-Tris Gel) and separated with an invitrogen NuPAGE electrophoresis system. The gel was silver-stained, gel fragments corresponding to the bands indicated were excised and destained using the SilverSNAPィ Stain for Mass Spectrometry (Pierce). Then proteins were trypsinized using the in-gel digestion method. Digested proteins were analyzed on an LTQ-Orbitrap (Thermo Scientific) by the nanoLC-ESI-MS/MS technique. Peptides were identified by the SEQUEST algorithm and filtered with a high confidence threshold (Protein false discovery rate < 1%, 2 peptides per protein minimum).
- Reviewer comment
- Esther Chan: Needs full peptide list to be included
- Submitted by
- Kathrina Onate
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG004558
- Download
- ZKSCAN1_final.pdf