ENCAB000AFF
Antibody against Homo sapiens CHD1, Mus musculus CHD1
Homo sapiens
K562, HeLa-S3
characterized to standards
Homo sapiens
MCF-7
characterized to standards with exemption
Homo sapiens
any cell type or tissue, A549
partially characterized
Mus musculus
any cell type or tissue
partially characterized
Homo sapiens
GM12878
not characterized to standards
- Status
- released
- Source (vendor)
- Novus
- Product ID
- NB100-60411
- Lot ID
- A1
- Characterized targets
- CHD1 (Homo sapiens), CHD1 (Mus musculus)
- Host
- rabbit
- Clonality
- polyclonal
- Purification
- affinity
- Antigen description
- The epitope recognized by this antibody maps to a region between residue 1660 and 1710 of human chromodomain helicase DNA binding protein 1 using the numbering given in entry NP_001261.2 (GeneID 1105).
- External resources
Characterizations
CHD1 (Homo sapiens)
A549
not submitted for review by lab
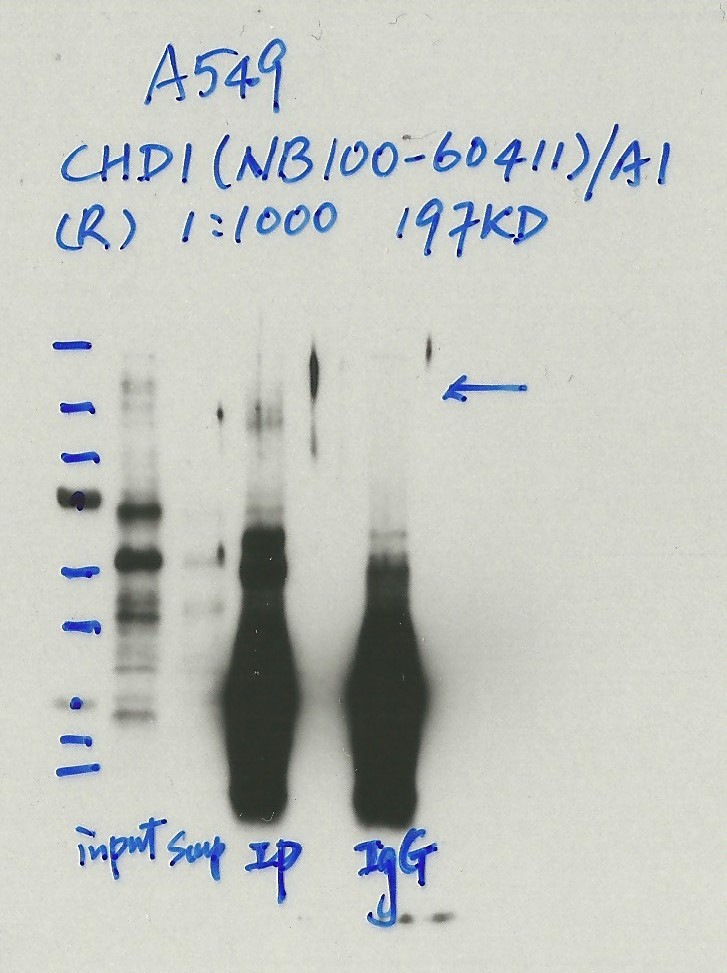
- Caption
- Immunoprecipitation was performed on nuclear extracts from the cell line: A549 using the antibody NB100-60411. The image shows western blot analysis of input, flowthrough, immunoprecipitate, and mock immunoprecipitate using IgG. Target molecular weight: 197.0.
- Submitted by
- Nathaniel Watson
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG006996
- Download
- #1142 A549 CHD1(NB100-60411).jpg
CHD1 (Homo sapiens)
Method: immunoprecipitation followed by mass spectrometry
compliant
- Caption
- IP followed by mass spectrometry: Briefly, K562 whole cell lysates were immunoprecipitated using NB100-60411, and the IP fraction was loaded on a 10% polyacrylamide gel (NuPAGE Bis-Tris Gel) and separated with an invitrogen NuPAGE electrophoresis system. The gel was silver-stained, gel fragments corresponding to the bands indicated were excised and destained using the SilverSNAP Stain for Mass Spectrometry (Pierce). Then proteins were trypsinized using the in-gel digestion method. Digested proteins were analyzed on an LTQ-Orbitrap (Thermo Scientific) by the nanoLC-ESI-MS/MS technique. Peptides were identified by the SEQUEST algorithm and filtered with a high confidence threshold (Protein false discovery rate < 1%, 2 peptides per protein minimum). We report 20 proteins identified in band A, of which 15 are also identified in a control immunoprecipitation. CHD1 is by far the most abundant of the specifically immunoprecipitated proteins (38 peptides). CHD1 is also identified in minor bands B (8 peptides) and C (7 peptides) and is the only chromosome associated protein from either band that is specifically immunoprecipitated. Based on these observations, the major band is likely due to the presence of immunoprecipitated CHD1 and NB100-60411 meets the ENCODE standard for validation by this criterion.
- Submitted by
- Kathrina Onate
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG004558
- Download
- CHD1_final_AFF Sheet1.pdf
CHD1 (Homo sapiens)
MCF-7
exempt from standards
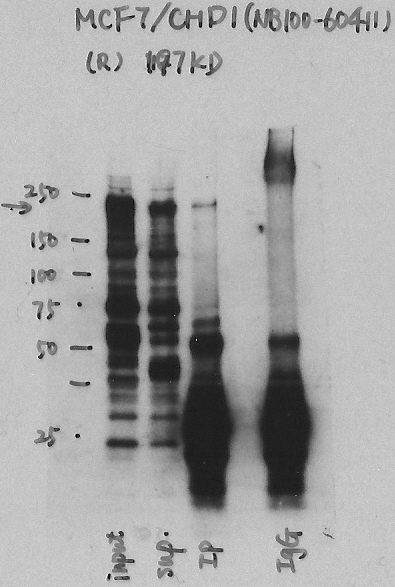
- Caption
- Immunoprecipitation was performed on nuclear extracts from the cell line: MCF-7, using the antibody NB100-60411. The blot shows western blot analysis of input, flowthrough, immunoprecipitate and mock immunoprecipitate using IgG.
- Submitter comment
- We showed that the CDH1 is present in 3 bands on the gel with mass spec on K562 cells. I think in the IP on MCF-7 cells we can see band A and band C with band C being stronger than band A.
- Reviewer comment
- Major band signal corresponds to <50% but IP-MS in K562 shows that protein can be detected in the strongest band (band C in the MS).
- Submitted by
- Denis Salins
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG006996
- Download
- Exp.810_CHD1.jpg
CHD1 (Homo sapiens)
Method: immunoprecipitation
not reviewed
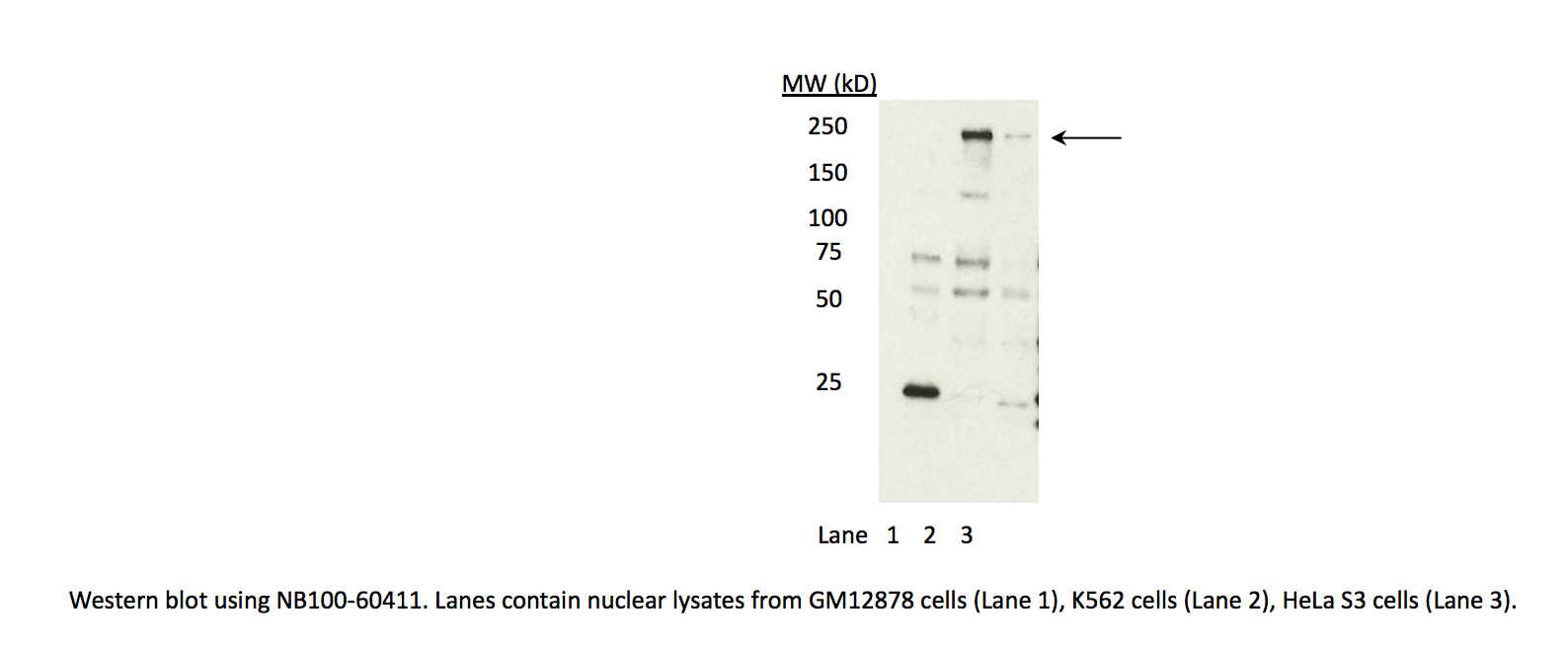
- Caption
- In an immunoblot probed with NB100-60411, we observe a major band consistent with the expected size of CHD1 (197kD) in lysates from cell lines K562 and HeLaS3. The band is also observed with lysates from GM12878 cells, but at low abundance. This band is specifically immunoprecipitated from K562 cells, as are several minor bands (likely degradation products) and each is identified by mass spectrometry as CHD1 (see second validation) Therefore, NB100-60411 meets this criterion for validation.
- Submitted by
- Michael Snyder
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG004558
CHD1 (Homo sapiens)
Method: immunoprecipitation followed by mass spectrometry
not reviewed
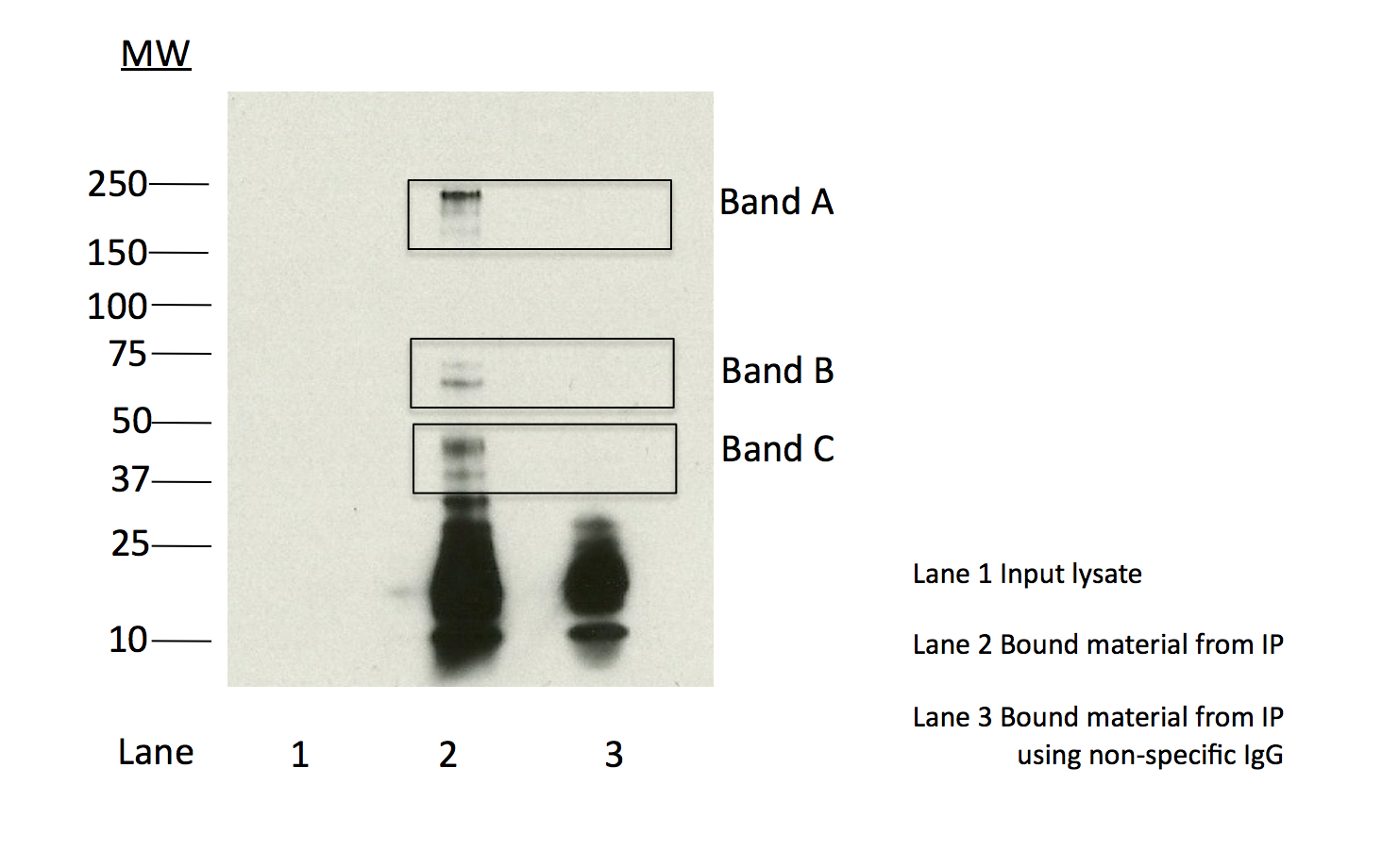
- Caption
- Immunoprecipitation of CHD1 from K562 cells using NB100-60411. Lane 1: input nuclear lysate, Lane 2: material immunoprecipitated with ab87525, Lane 3: material immunoprecipitated using control IgG. Band A, the most abundant band and consistent with the expected size of CHD1, along with minor bands B and C were excised from the gel and subject to analysis by mass spectrometry. IP followed by mass spectrometry: Briefly, K562 whole cell lysates were immunoprecipitated using NB100-60411, and the IP fraction was loaded on a 10% polyacrylamide gel (NuPAGE Bis-Tris Gel) and separated with an invitrogen NuPAGE electrophoresis system. The gel was silver-stained, gel fragments corresponding to the bands indicated were excised and destained using the SilverSNAP Stain for Mass Spectrometry (Pierce). Then proteins were trypsinized using the in-gel digestion method. Digested proteins were analyzed on an LTQ-Orbitrap (Thermo Scientific) by the nanoLC-ESI-MS/MS technique. Peptides were identified by the SEQUEST algorithm and filtered with a high confidence threshold (Protein false discovery rate < 1%, 2 peptides per protein minimum). We report 20 proteins identified in band A, of which 15 are also identified in a control immunoprecipitation. CHD1 is by far the most abundant of the specifically immunoprecipitated proteins (38 peptides). CHD1 is also identified in minor bands B (8 peptides) and C (7 peptides) and is the only chromosome associated protein from either band that is specifically immunoprecipitated. Based on these observations, the major band is likely due to the presence of immunoprecipated CHD1 and NB100-60411 meets the ENCODE standard for validation by this criterion.
- Submitted by
- Michael Snyder
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG004558
CHD1 (Homo sapiens)
K562
compliant
- Caption
- Immunoprecipitation of CHD1 from K562 cells using NB100-60411. Lane 1: input nuclear lysate, Lane 2: material immunoprecipitated with ab87525, Lane 3: material immunoprecipitated using control IgG. Band A, the most abundant band and consistent with the expected size of CHD1, along with minor bands B and C were excised from the gel and subject to analysis by mass spectrometry. IP followed by mass spectrometry: Briefly, K562 whole cell lysates were immunoprecipitated using NB100-60411, and the IP fraction was loaded on a 10% polyacrylamide gel (NuPAGE Bis-Tris Gel) and separated with an invitrogen NuPAGE electrophoresis system.
- Submitted by
- Kathrina Onate
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG004558
- Download
- IP:MS Snyder AFF.png
CHD1 (Mus musculus)
Method: ChIP-seq comparison
not reviewed
- Caption
- This antibody has been validated by IP-Mass Spec analysis in K562 cells. Please see the validation document for this antibody in human cell lines for details.
- Submitted by
- Michael Snyder
- Lab
- Michael Snyder, Stanford
- Grant
- RC2HG005602
- Download
- mouse_CHD1_validation_Snyder.pdf
CHD1 (Mus musculus)
Method: immunoprecipitation
not reviewed
- Caption
- A band of ~240 kD (expected size of Chd1) is efficiently immunoprecipitated from MEL and CH12 lysates by NB100-60411. This antibody has been previously validated by immunblotting in human cell lines K562, GM12878 and HeLaS3 as well as by Mass Spec analysis of proteins immunioprepcipitated by this antibody and this validation information has been submitted. In combination with the previously submitted data, NB100-60411 meets the ENCODE criteria for validation in murine cell lines CH12 and MEL.
- Submitted by
- Michael Snyder
- Lab
- Michael Snyder, Stanford
- Grant
- RC2HG005602
- Download
- mouse_CHD1_validation_Snyder.pdf
CHD1 (Homo sapiens)
GM12878K562HeLa-S3
compliant
- Caption
- In an immunoblot probed with NB100-60411, we observe a major band consistent with the expected size of CHD1 (197kD) in lysates from cell lines K562 and HeLaS3. The band is also observed with lysates from GM12878 cells, but at low abundance. This band is specifically immunoprecipitated from K562 cells, as are several minor bands (likely degradation products) and each is identified by mass spectrometry as CHD1 (see second validation) Therefore, NB100-60411 meets this criterion for validation.
- Submitted by
- Kathrina Onate
- Lab
- Michael Snyder, Stanford
- Grant
- U54HG004558
- Download
- WB Snyder AFF.png