ENCAB000BIM
Antibody against Homo sapiens RUNX1
Homo sapiens
K562, HepG2
characterized to standards with exemption
- Status
- released
- Source (vendor)
- Sigma
- Product ID
- WH0000861M6
- Lot ID
- 11060-2C10
- Characterized targets
- RUNX1 (Homo sapiens)
- Host
- mouse
- Clonality
- monoclonal
- Isotype
- IgGκ
- Antigen description
- RUNX1 (NP_001001890, a.a. 210-311) partial recomb protein w/ GST tag.MW of GST tag alone is 26 kDa.
- External resources
Characterizations
RUNX1 (Homo sapiens)
Method: motif enrichment
compliant
- Caption
- The motif for target RUNX1 is represented by the attached position weight matrix (PWM) derived from file ENCFF406HKZ. Motif enrichment analysis was done by Dr. Zhizhuo Zhang (Broad Institute, Kellis Lab) using known motifs (http://compbio.mit.edu/encode-motifs/) and previously published ChIP-seq data (http://www.broadinstitute.org/~zzhang/motifpipeline/data/TrainSetInfo.txt). The accept probability score of the given transcription factor was calculated using a Bayesian approach. This analysis also includes three motif enrichment scores, computed by overlapping the motif instances with the given ChIP-seq peak locations. For more information on the underlying statistical methods, please see the attached document. Accept probability score: 0.95806062559, Global enrichment Z-score: 3.31078939753, Positional bias Z-score: 3.55529392607, Peak rank bias Z-score: 4.14003383256, Enrichment rank: 2.0.
- Submitted by
- Aditi Narayanan
- Lab
- Richard Myers, HAIB
- Grant
- U54HG006998
- Download
- ENCAB000BIM.pdf
RUNX1 (Homo sapiens)
not compliant
- Caption
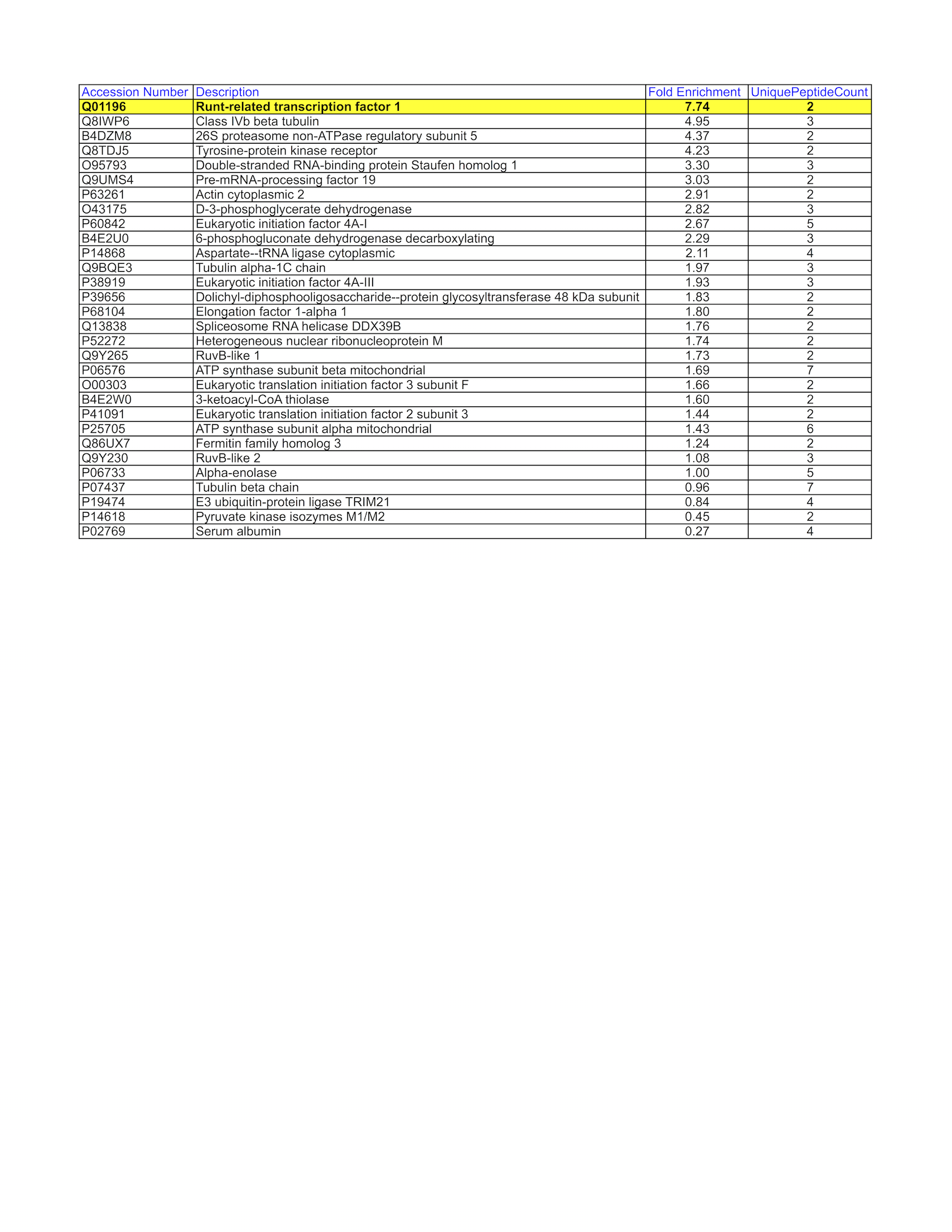
- K562 whole cell lysate was immunoprecipitated using the primary antibody (Sigma; WH0000861M6). The IP fraction was loaded on a 12% Bio-Rad TGX gel and separated with the Bio-Rad Tetra Cell system. A gel fragment (rectangle outline) corresponding to the band indicated on the Coomassie Blue stained gel image was excised and sent to the University of Alabama at Birmingham Cancer Center Mass Spectrometry/Proteomics Shared Facility. Analysis of gel fragment 1 from K562: The sample was analyzed on a LTQ XL Linear Ion Trap Mass Spectrometer by LC-ESI-MS/MS. Peptides were identified using SEQUEST tandem mass spectral analysis with probability based matching at p < 0.05. SEQUEST results were reported with ProteinProphet protXML Viewer (TPP v4.4 JETSTREAM) and filtered for a minimum probability of 0.9. All protein hits that met these criteria were reported, including common contaminants. Fold enrichment for each protein reported was determined using a custom script based on the FC-B score calculation from the reference Mellacheruvu et al., 2013. The CRAPome: a contaminant repository for affinity purification mass spectrometry data. Nat. Methods. 10(8):730-736. Doi:10.1038/nmeth.2557. The target protein, RUNX1, was not identified based on IP-Mass Spectrometry.
- Submitter comment
- Target of interest is not within the top 20 of the list by fold enrichment
- Submitted by
- Mark Mackiewicz
- Lab
- Richard Myers, HAIB
- Grant
- U54HG004576
- Download
- RUNX1-1.png
RUNX1 (Homo sapiens)
compliant
- Caption
- K562 whole cell lysate was immunoprecipitated using the primary antibody (Sigma; WH0000861M6). The IP fraction was loaded on a 12% Bio-Rad TGX gel and separated with the Bio-Rad Tetra Cell system. A gel fragment (rectangle outline) corresponding to the band indicated on the Coomassie Blue stained gel image was excised and sent to the University of Alabama at Birmingham Cancer Center Mass Spectrometry/Proteomics Shared Facility. Analysis of gel fragment 2 from K562: The sample was analyzed on a LTQ XL Linear Ion Trap Mass Spectrometer by LC-ESI-MS/MS. Peptides were identified using SEQUEST tandem mass spectral analysis with probability based matching at p < 0.05. SEQUEST results were reported with ProteinProphet protXML Viewer (TPP v4.4 JETSTREAM) and filtered for a minimum probability of 0.9. All protein hits that met these criteria were reported, including common contaminants. Fold enrichment for each protein reported was determined using a custom script based on the FC-B score calculation from the reference Mellacheruvu et al., 2013. The CRAPome: a contaminant repository for affinity purification mass spectrometry data. Nat. Methods. 10(8):730-736. Doi:10.1038/nmeth.2557. The target protein, RUNX1, was identified as the 1st ranked enriched protein and the 1st ranked transcription factor based on IP-Mass Spectrometry.
- Submitted by
- Mark Mackiewicz
- Lab
- Richard Myers, HAIB
- Grant
- U54HG006998
- Download
- RUNX1-2.png
RUNX1 (Homo sapiens)
K562
not reviewed
- Submitted by
- Mark Mackiewicz
- Lab
- Richard Myers, HAIB
- Grant
- U54HG006998
- Download
- RUNX1_IP-Commassie1.png
RUNX1 (Homo sapiens)
K562HepG2
exempt from standards
- Caption
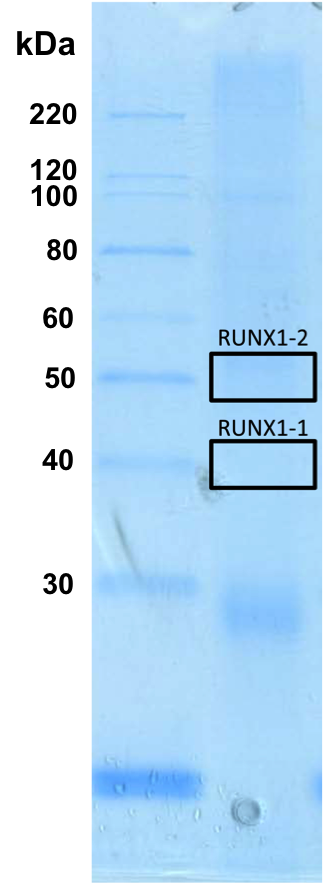
- Whole cell lysates of K562 and HepG2 were immunoprecipitated using the primary antibody (Sigma; WH0000861M6). The IP fraction was separated on a 12% acrylamide gel with the Bio-Rad PROTEAN II xi system. After separation, the samples were transferred to a nitrocellulose membrane with an Invitrogen iBlot system. The membrane was probed with the primary antibody (same as that used for IP) and a secondary HRP-conjugated antibody. The resulting bands were visualized with SuperSignal West Femto Solution (Thermo Scientific). Protein Marker (PM) is labeled in kDa. Two bands were detected at ~40 and 50 kDa. Expected size ~48 KDa.
- Submitter comment
- --
- Reviewer comment
- The band indicated is not 50% of the overall signal
- Submitted by
- Mark Mackiewicz
- Lab
- Richard Myers, HAIB
- Grant
- U54HG006998
- Download
- RUNX1_IP-WB.png
RUNX1 (Homo sapiens)
K562HepG2
not compliant
- Caption
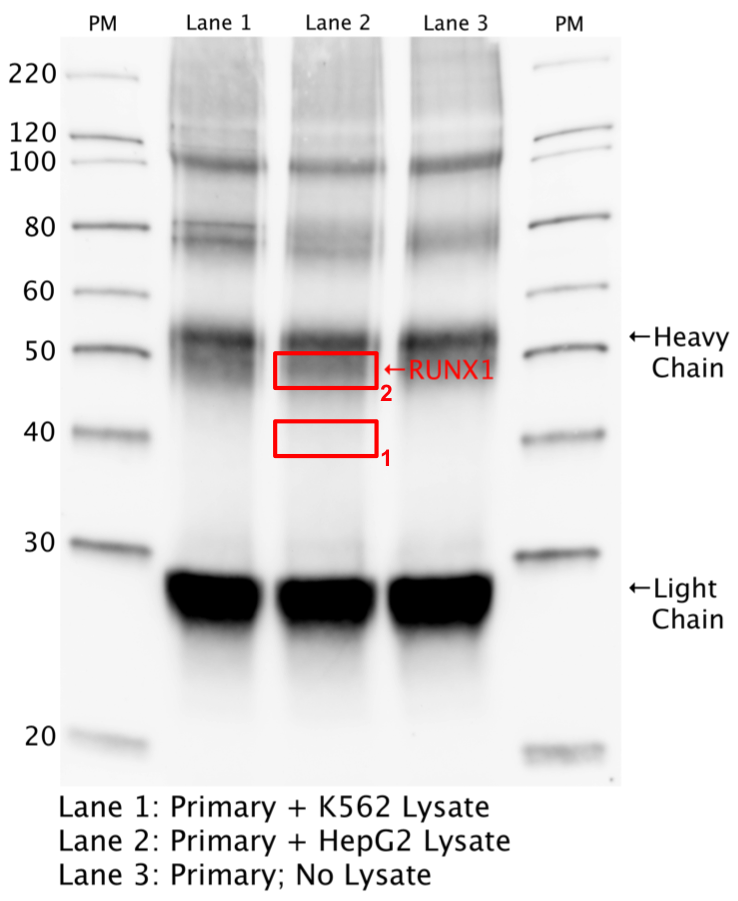
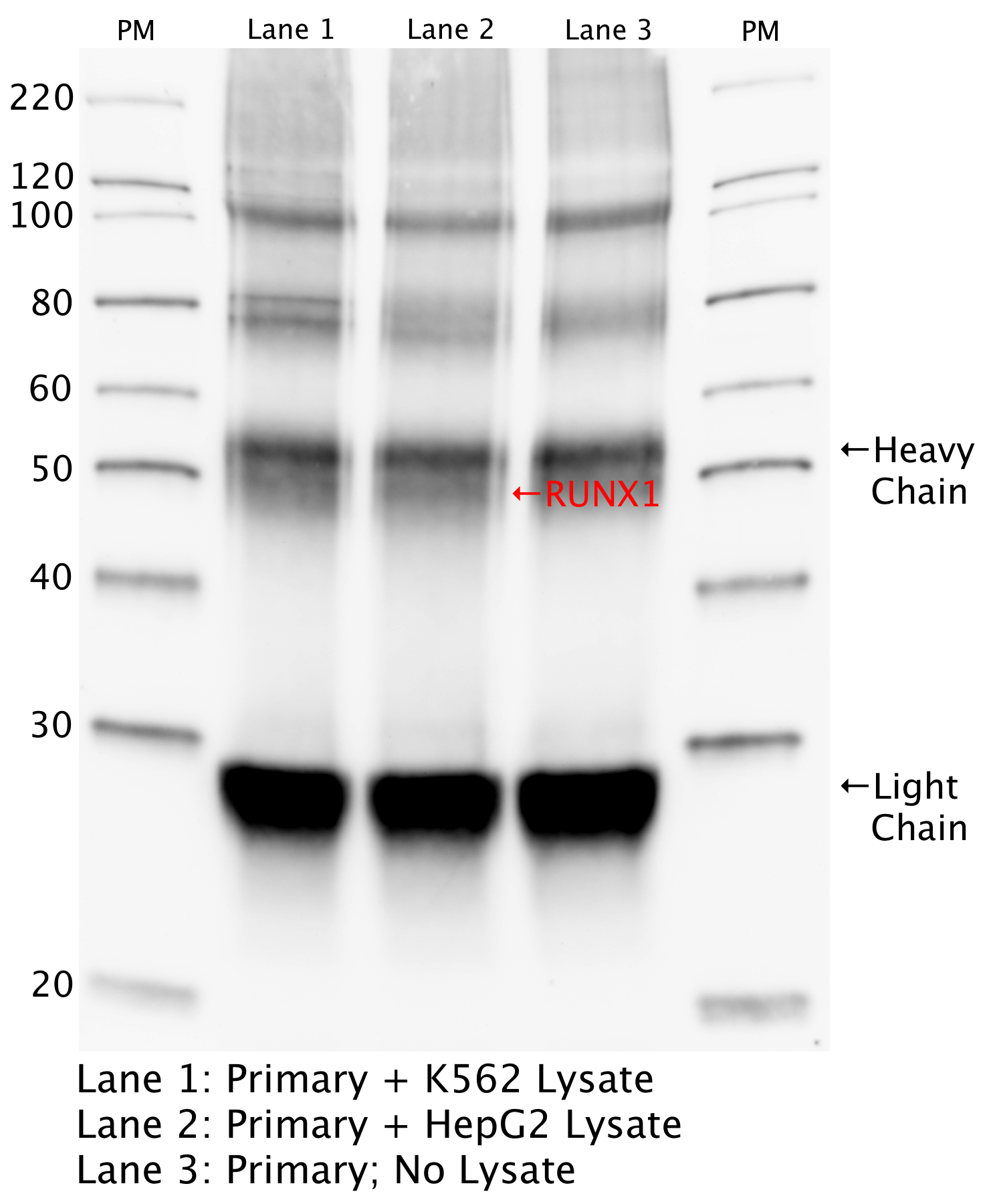
- An immunoprecipitation followed by Western blot was performed with Sigma WH0000861M6 (lot 11060-2C10) antibody against RUNX1 in K562 and HEPG2 whole cell lysate along with a control IP witn no lysate. The expected size of RUNX1 is 20-52 kDa. Bands of ~40 and 52 kDa were detetected. The experiment was repeated and these bands were analyzed by mass spec in the secondary validation.
- Submitted by
- Flo Pauli-Behn
- Lab
- Richard Myers, HAIB
- Grant
- U54HG006998